Additive manufacturing in orthopedics – lattice structures
Written by Jonathan Buckley
Published on August 5, 2020
See how Amplify Additive focuses on fusion and osseointegration to manufacture implants.

54 stacked acetabular hip cups printed using the ArcamEBM Q10Plus platform.
We at Amplify Additive are focused on fusion and bone ingrowth (i.e. osseointegration) when we manufacture implants, as that is a critical function that must be optimized at all costs. By definition osseointegration is the connection between bone and the surface of an implant; therefore the degree and rate of fusion directly correlates to a more effective implant for the end user.
In traditionally manufactured orthopedic implants (i.e. fully machined implants), coatings such as TPA and sintered coatings are applied after machining to ensure osseointegration occurs between the implant and the part. Coatings such as these have been applied since the 1970’s and while they have proven effective in promoting bone to implant integration, implant failure and the need for revision surgeries have been known to occur from time to time. Coatings such as TPA require line of sight to coat an implant, which can be difficult with inner surface features and may require multiple applications to get the desired thickness. Sintered coatings require an additional heat treatment for proper adhesion, which also can weaken the overall strength of the implant.
With the newest innovations from additive manufacturing, we at Amplify Additive use 3D printed lattice structures to promote improved osseointegration vs traditional implants. Lattice structures are 3D printed as part of the implant design, allowing for seamless integration between both. These lattice structures have the appearance of a porous grid, comprised of thin struts overlapping with each other while maintaining small spacings in between which are known as pores. Unlike coatings, the pore size, strut size and % porosity can all be changed according to the individual implant needs, which allows us to generate the most effective implants to date.
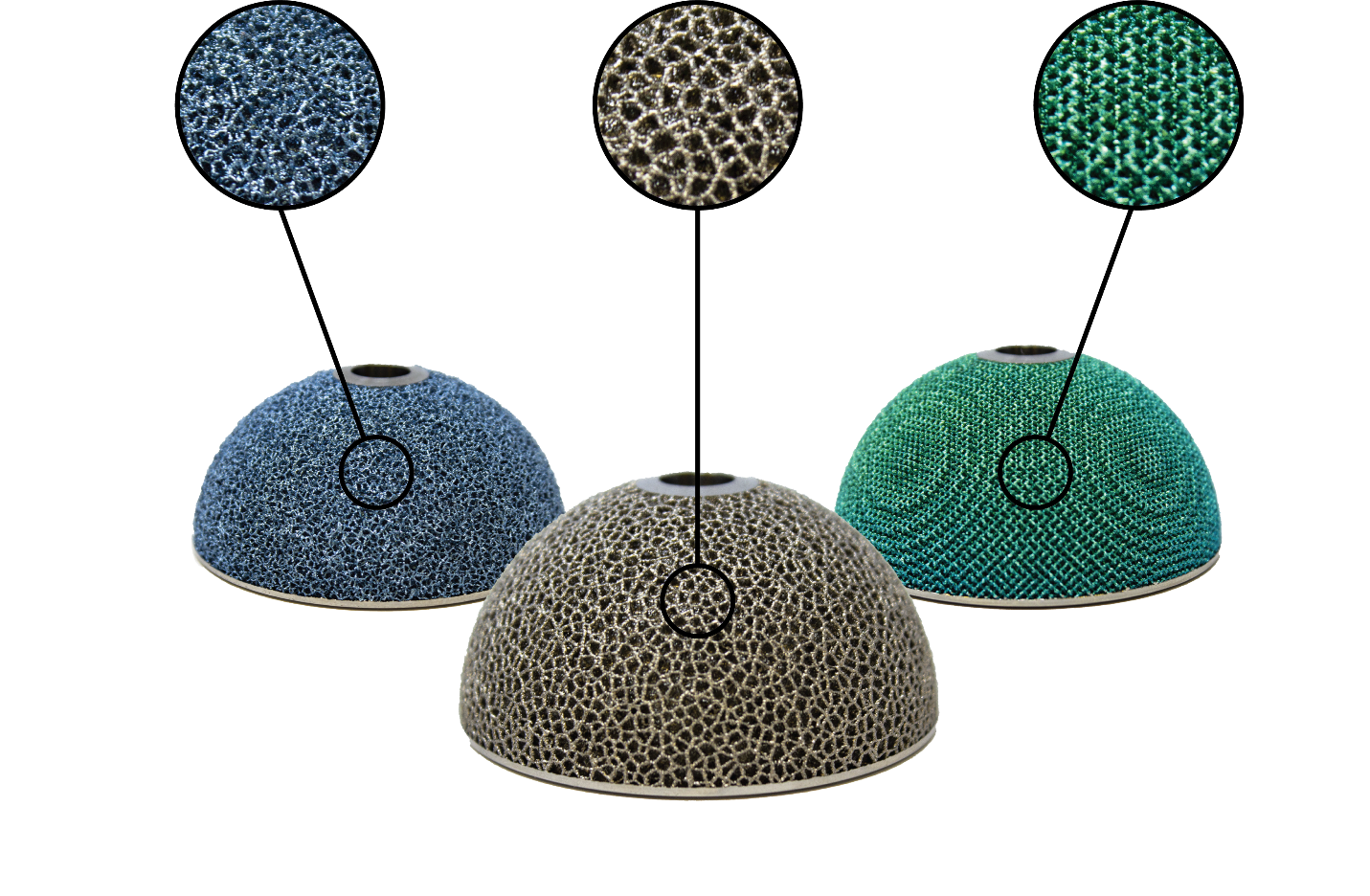
Stochastic, trabecular and engineered lattice structures printed using EBMEBtechnology.
Multiple research papers have found that these additive manufactured lattice structures offer better patient comfort, expedited cell proliferation, and stronger implants due to the lattice being directly implemented into the implant design. In one study comparing additive manufactured samples to TPS samples, it was found that the additively manufactured samples had significantly more bony ongrowth, ingrowth and through growth than the TPS samples.1 By implementing the improvements additive manufacturing can bring to implant design, a decrease in the occurrence of subsidence, pseudoarthrosis, stress-shielding and implant failure is expected to occur.1 These benefits are only one of many for the orthopedic industry to move towards metal 3D printed implants, as additive manufacturing can provide design freedom, improved mechanical properties, faster time to market and an efficient design cycle among other benefits to manufacturers, which translates to better patient outcomes.

Sample compression test coupons for lattice qualification.
To generate these lattice structures, a dedicated software is first needed to design the lattice as a 3D model. Multiple software suites are available in the marketplace, however we at Amplify Additive have found that nTop thus far has been the most comprehensive lattice generation software to date. nTop allows you to generate lattices through organized workflows, which results in better file control, especially when applying lattice structures between different part sizes and families. Additionally, nTop offers various lattice structures designs that can be generated, each of which that can be customized depending on the needs of the customer.
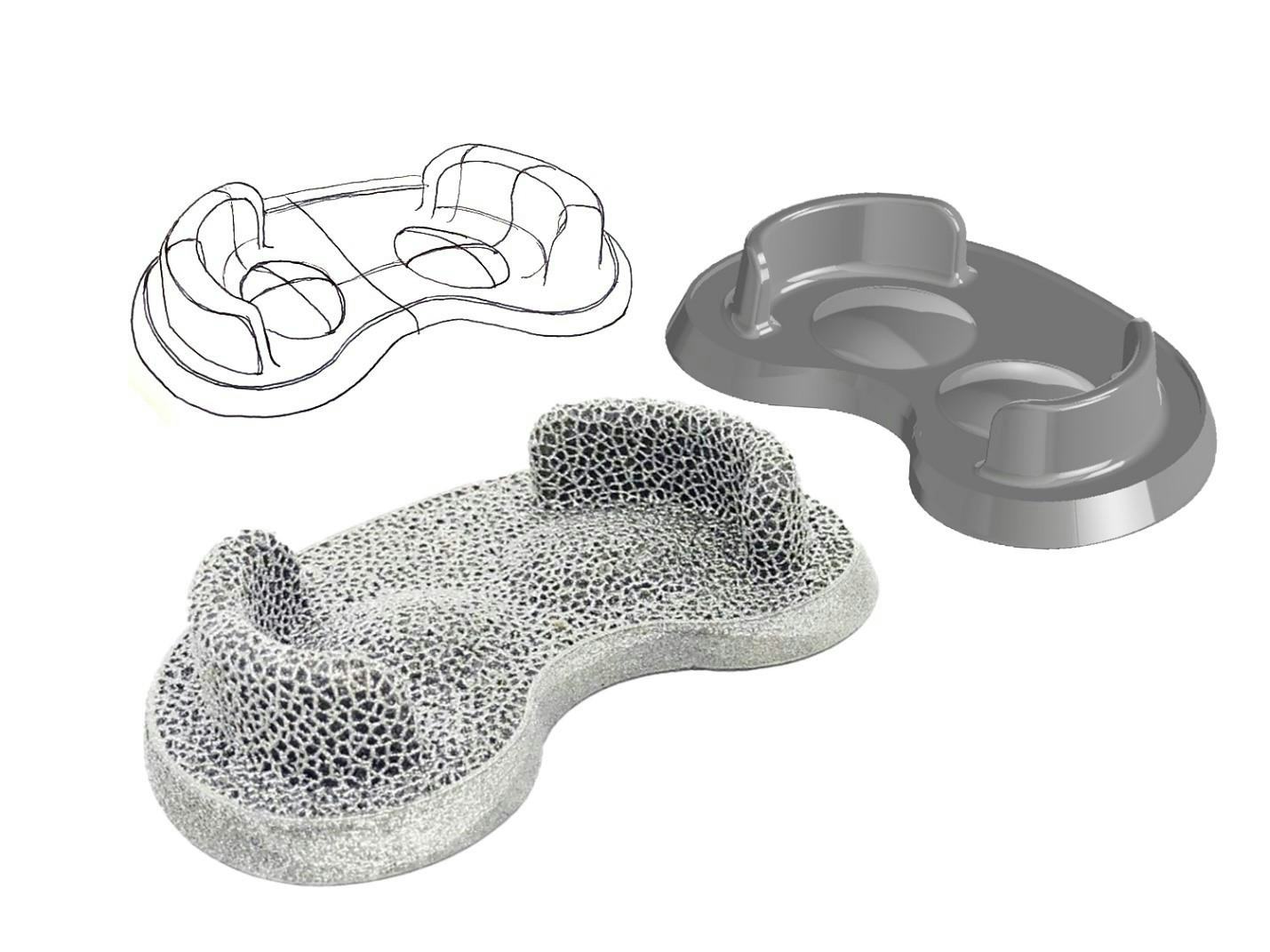
From concept to CAD to physical print.
Additive Manufacturing offers not only significant design freedoms when utilized to its fullest extent, but also a more effective implant manufacturing process. We at Amplify Additive strive to leverage the benefits of additive manufacturing to help deliver innovative solutions to the Orthopedic Implant Marketplace, and nTop is an effective tool to help us achieve the mission.
Amplify Additive is a Contract Additive Manufacturer that utilizes Electron Beam Melting (EBM) Technology in the production of Orthopedic Implants. We lead the industry in not only our experience utilizing Additive Manufacturing in a production setting, but also with providing regulatory guidance to help bring additive manufactured products through FDA 510(k) submission and clearance to the market. Let us know how we can assist you in your additive journey. www.amplifyadditive.com
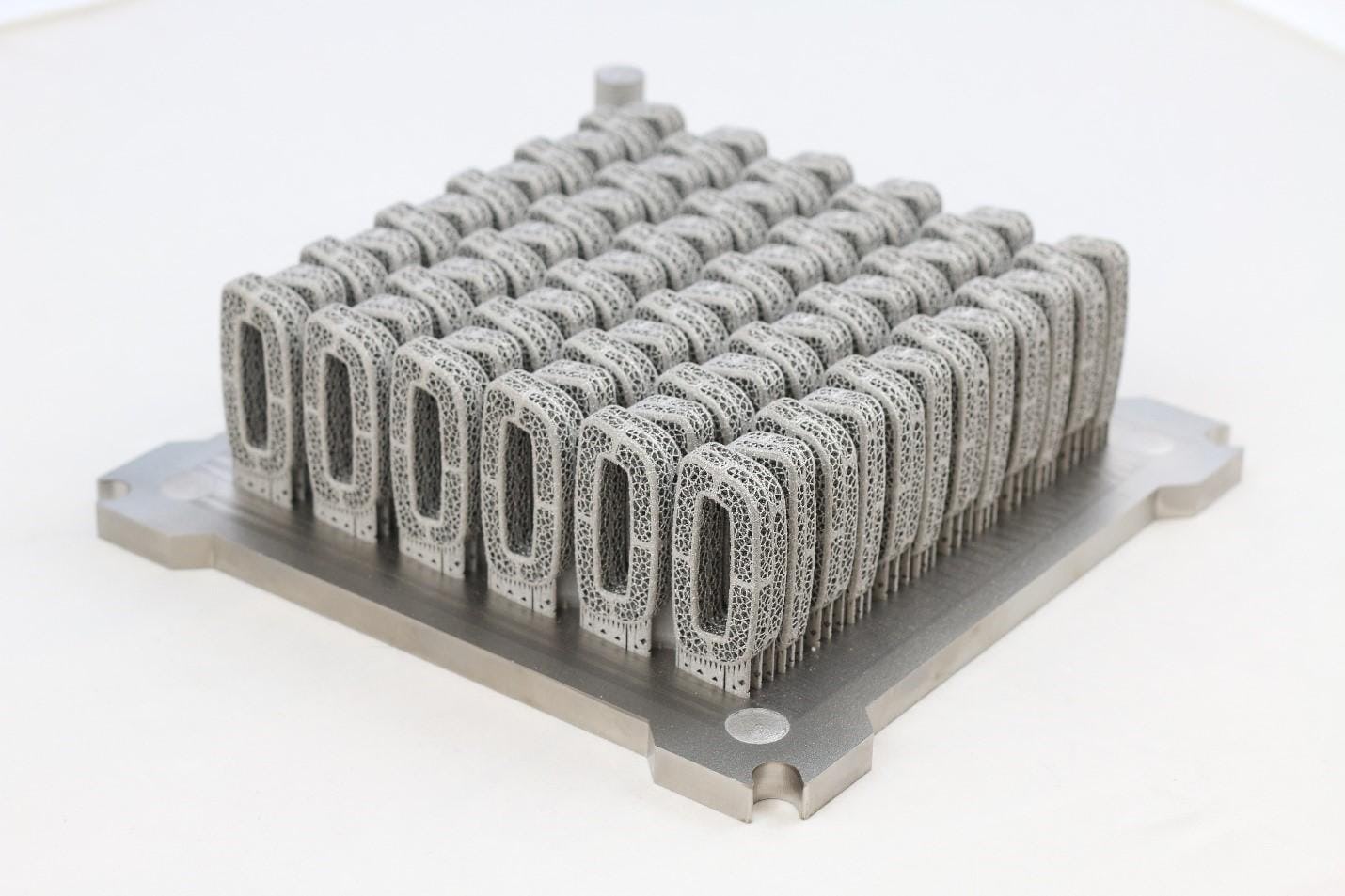
Single layer build of Ti6Al4V 3D printed spinal implants printed with EBM technology.
References:
- MacBarb, Regina F et al. “Fortifying the Bone-Implant Interface Part 1: An In Vitro Evaluation of 3D-Printed and TPS Porous Surfaces.” International journal of spine surgeryvol. 11,3 15. 1 Jun. 2017, doi:10.14444/4015

Jonathan Buckley
Jonathan is the Senior AM Applications Engineer at Amplify Additive. He supports the team by managing Electron Beam Melting (EBM) technology in the production of orthopedic and medical devices. Jonathan has extensive experience in the additive manufacturing field and applying EBM technology from his prior position as an Applications Engineer at GE Additive, and now provides this expertise to Amplify Additive.
Related content
- GUIDE
Download: Advanced design software and additive manufacturing for personalized implants

- GUIDE
Download: Design Automation for Medical Devices

- VIDEO
Design a spooky Halloween candy bowl in nTop

- ARTICLE
Improving the biomechanical profile of additive hip implants with Field Optimization
- ARTICLE
Design at scale with nTop Automate