Next-generation medical implant design, production, and validation using additive manufacturing and nTop at Beijing DPR - Chinese

video: Next-generation medical implant design, production, and validation using additive manufacturing and nTop at Beijing DPR - Chinese
Published on June 17, 2021
*This webinar is in Chinese.
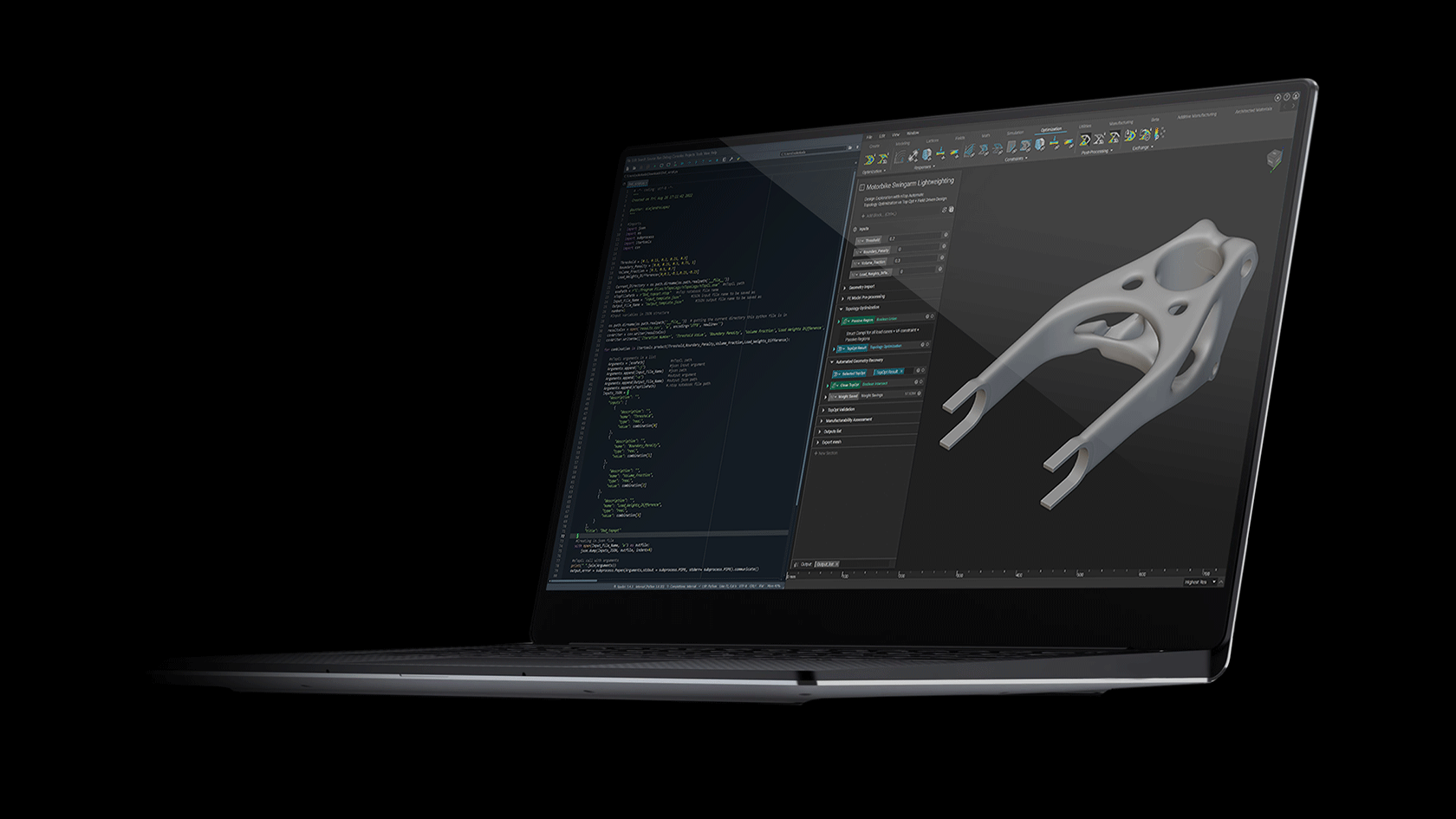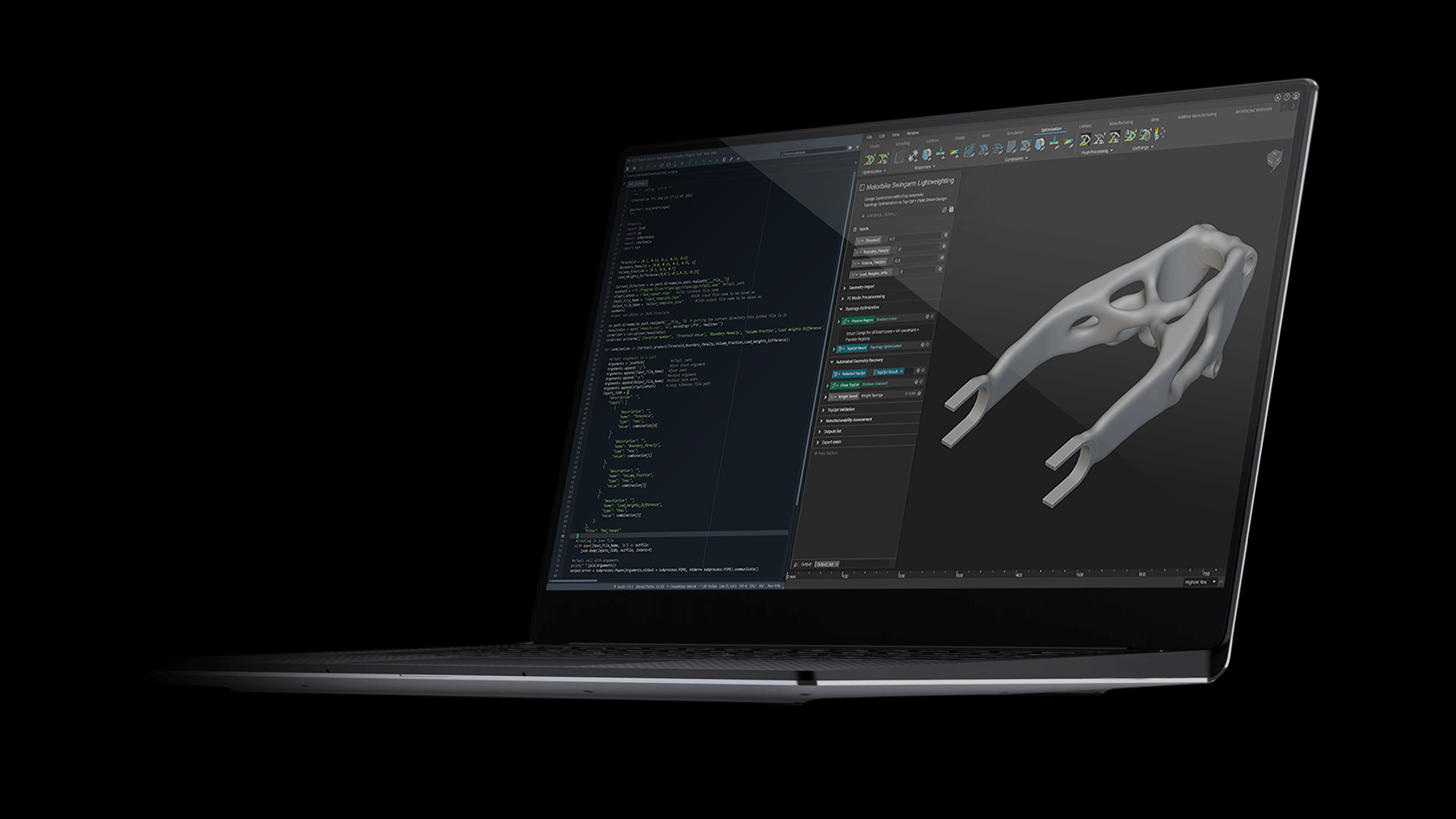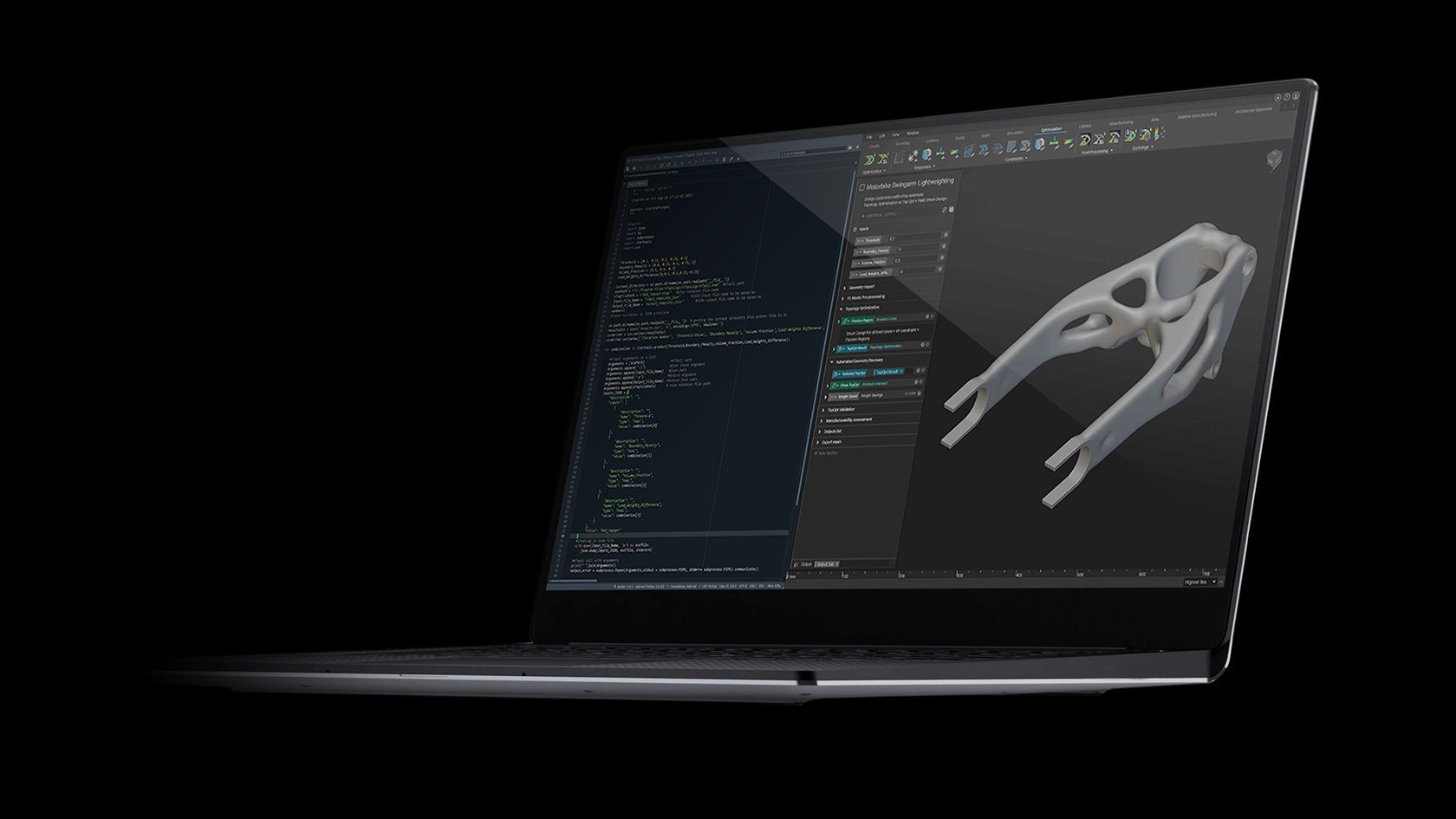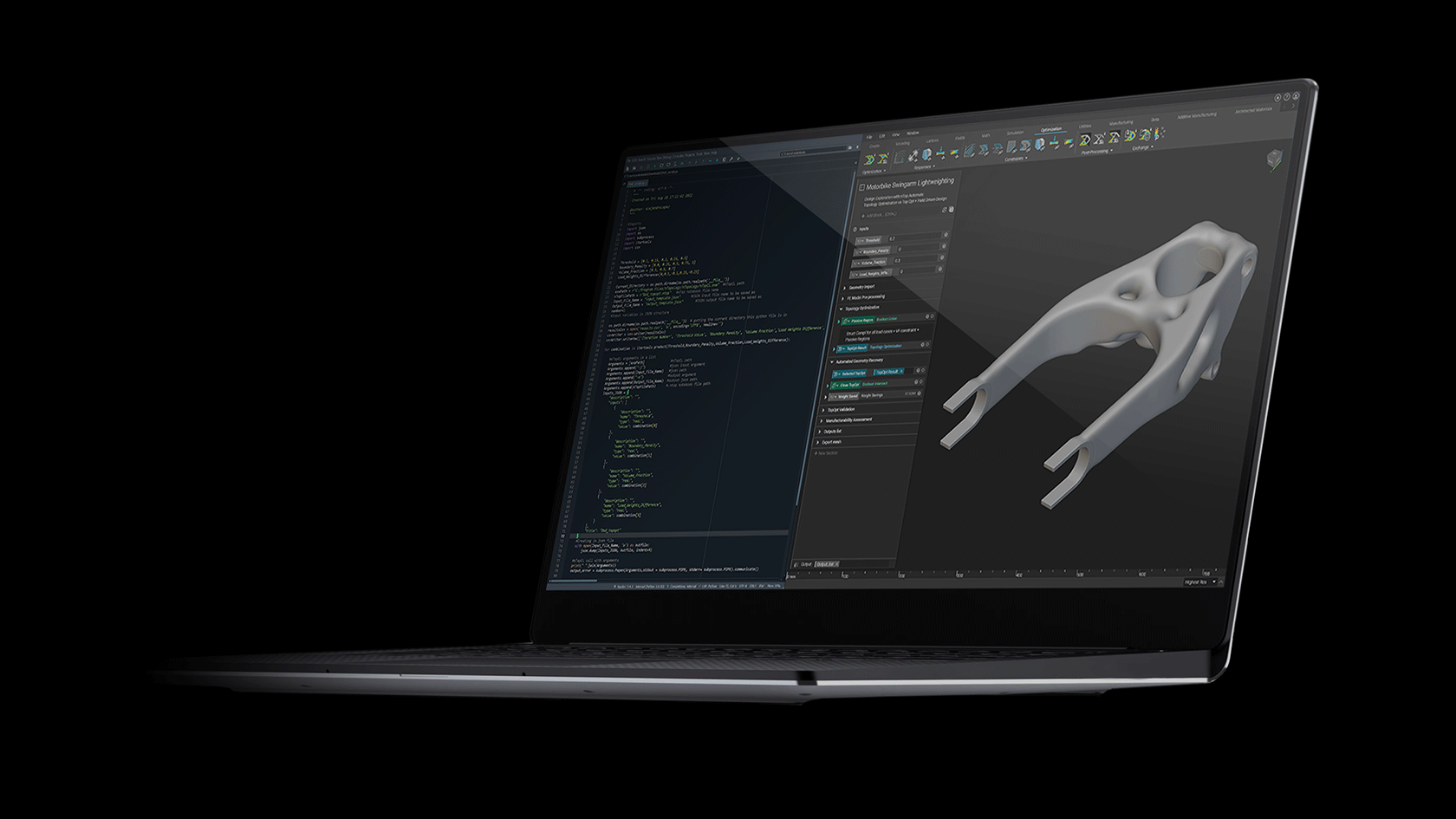
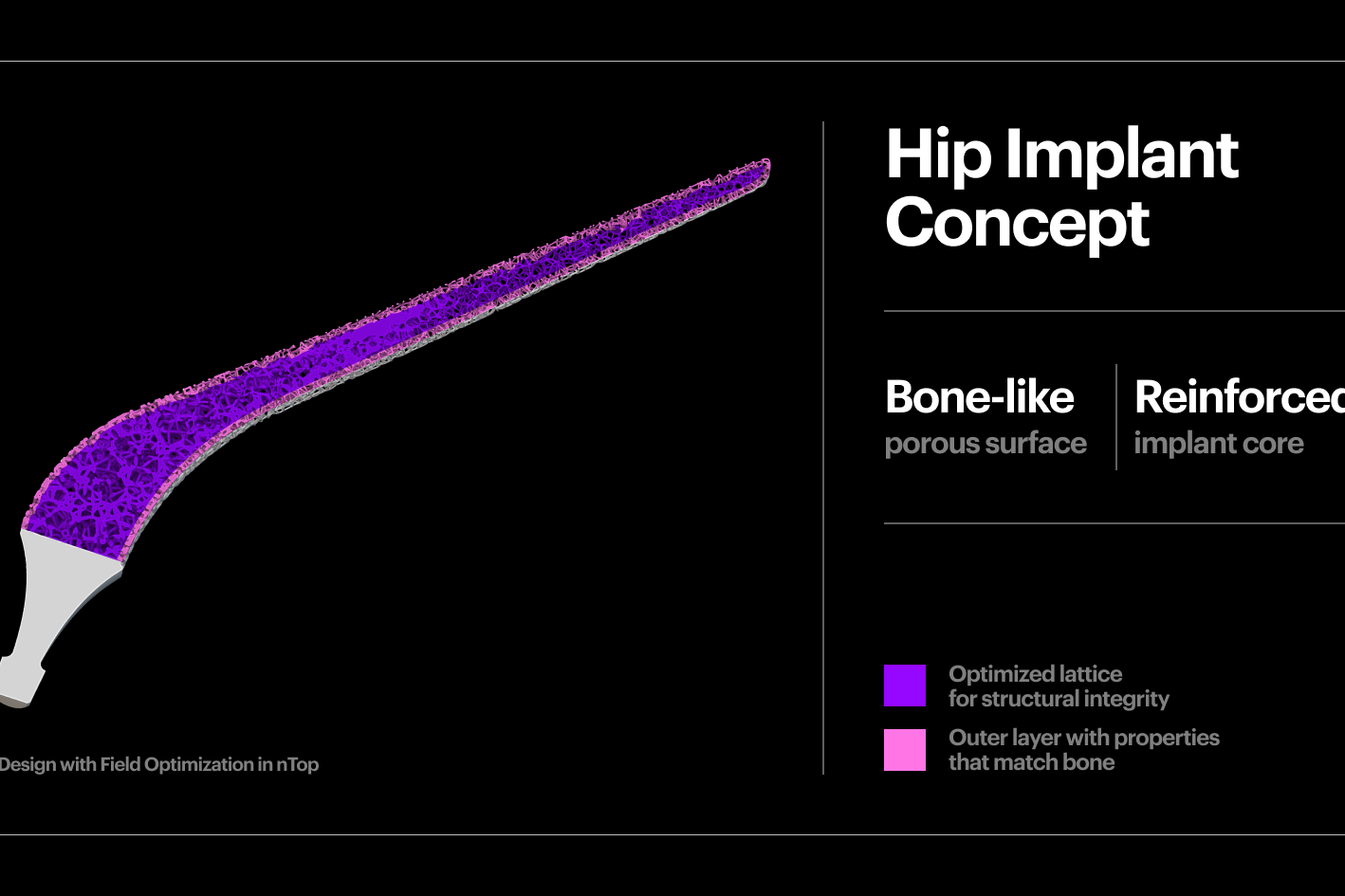
One of the first commercially viable use cases of additive manufacturing (AM) is the production of medical implants. AM enables the production of structures that promote bone growth into the implant which improves the mechanical bond between the bone and implant. This leads to an over better patient experience due to improved efficacy and longevity of the implant. These osseointegrative structures are not manufacturable by any other means than AM.
This webinar will demonstrate how these structures are designed in nTop and how the software gives engineers full control of every aspect of the design. Topics include Lattice Design, Porosity Specification, Lattice-to-Solid Blending, and Automation.
We will also discuss how these implants are produced with Additive Manufacturing at Beijing DPR. Topics include material verification, production parameter optimization, part verification, and post-processing. We will conclude with a live Q&A section.
Watch this webinar and learn:
- What is important in the Design & Production of additively manufactured medical implants.
- How nTop and Beijing DPR leverage implicit modeling & manufacturing knowledge to produce medical implants.
- How these design & production methods create better products and overall patient outcomes.
Related content
- GUIDE
Download: Advanced design software and additive manufacturing for personalized implants

- GUIDE
Download: Design Automation for Medical Devices

- VIDEO
Design a spooky Halloween candy bowl in nTop

- ARTICLE
Improving the biomechanical profile of additive hip implants with Field Optimization
- ARTICLE
Design at scale with nTop Automate